Our Accreditations

WHO-GMP Certificate issued by CDSCO

INTERCERT CERTIFICATE - CERTIFICATE OF Food Safety Management System of Surya Herbal Limited
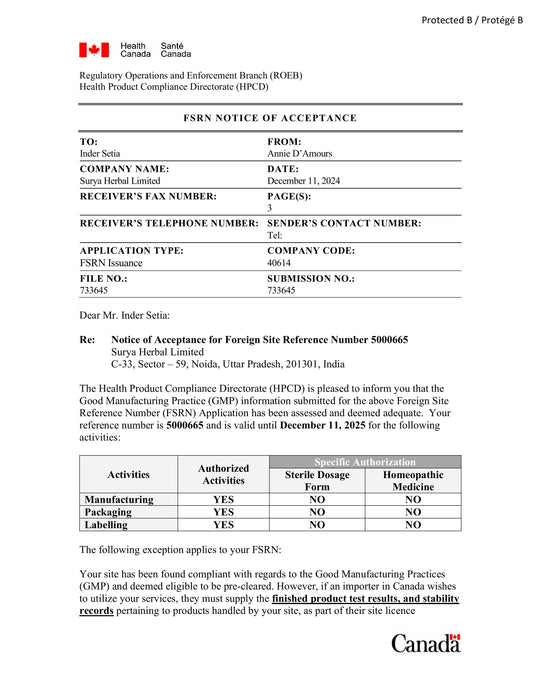
Foreign Site Reference Number (FSRN) Notice of Acceptance issued by Health Canada to Surya Herbal Limited

Registration of Factory of Medical Production by Kurdistan Regional Government-Iraq

Certificate for Good Manufacturing Practices(GMP)

Certification- ISO 22000: 2018 by Intercert, Accredited by IAS (International Accreditation Services) Inc. USA

Certification- ISO 9001:2015, Accredited by SSC (Statistical Society of Canada) Inc. USA.

Certification - ISO 13485:2016, Accredited by United Accreditation Foundation,

Halal Registration Certificate, Accredited by eiaci (Emirates International Accreditation Centre)
HBN-016. Certification by Jamiat Ulama-i-Hind Halal Trust,

AYUSH PREMIUM MARK SCHEME (adheres to WHO GMP
based system) Accredited by NABCB, Ayush
premium, QCI.

Manufacturing Site Registration Certificate
issued by Ministry of Health & Prevention, UAE

EU Certificate-Certificate of Conformity, issued by Certification Centre, BALTSERT Ltd.
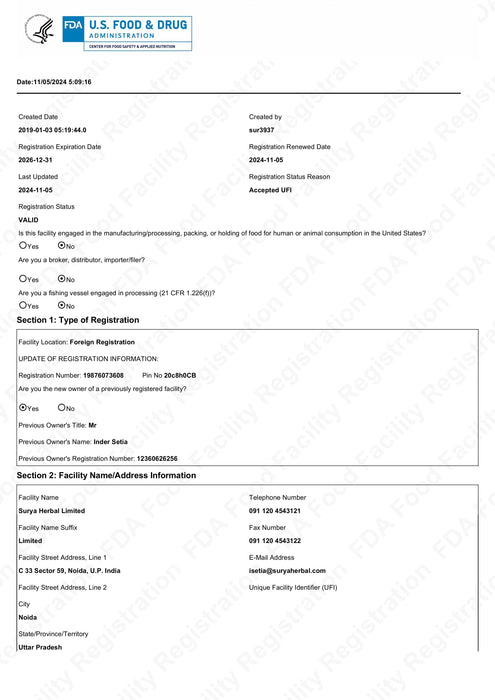
US Food Facility Registration Certificate, issued by U.S. Food & drug administration by Initial Registration
No. 19876073608 issued
dated 10.11.2022

Certificate/License for Manufacture for Sale Ayurvedic Drugs under From 15-D by Ayurveda by Licensing Authority/Director, Ayurvedic Services, Uttar Pradesh Lucknow & Certificate/Licebse no.: A-3077/2000 issued dated 16.03.2000

Certificate for Manufacturer & Export of Food or Health Supplements and Nutraceuticals by Food Safety and Standards (FSSAI) Authority of India is a statutory body under the administration of Ministry of Health and Family Welfare, Government of India & Certificate/Licebse no.: 10013051000815 issued dated 20.10.2022


